BASF, the world’s largest chemical producer based in Ludwigshafen, Germany, has partnered with ExxonMobil to develop low-emission hydrogen using methane pyrolysis technology. This collaboration aims to accelerate the production of cost-effective, clean hydrogen for industrial use. The companies have signed a joint development agreement and plan to build a demonstration plant in Baytown, Texas, to test the technology at scale.
BASF has been researching methane pyrolysis for several years with funding from Germany’s Federal Ministry of Research, Technology, and Space (BMFTR). By teaming up with ExxonMobil, the companies hope to combine expertise and bring this promising hydrogen solution closer to commercial reality.
BASF methane pyrolysis test facility at Ludwigshafen site

Methane Pyrolysis: Fueling a Low-Carbon Future
Methane pyrolysis is a process that splits methane—a major component of natural gas—into hydrogen and solid carbon using electricity. Unlike traditional hydrogen production methods, such as steam-methane reforming (SMR), methane pyrolysis does not produce CO2 during the reaction.
The process uses about five times less energy than water electrolysis and doesn’t require water, making it more efficient in many situations. Methane pyrolysis also benefits from existing natural gas infrastructure, so it can be deployed in multiple locations without major modifications.
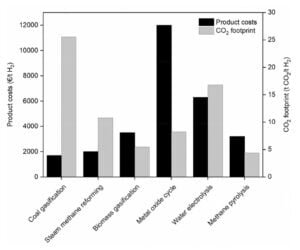
Product costs and CO2 footprint of different hydrogen production technologies
Key Benefits and Challenges
Methane pyrolysis can play an important role in the transition to a low-carbon economy. Hydrogen demand is expected to grow across industries, from chemicals and steel to transportation and energy storage. By producing hydrogen without direct CO2 emissions, methane pyrolysis can help industries meet decarbonization targets.
Hydrogen is a critical energy carrier and feedstock for the chemical industry. Solid carbon, the byproduct of methane pyrolysis, is also valuable. It can be used in steel and aluminum production, construction materials, and advanced carbon products like battery components.
Key advantages of methane pyrolysis include:
-
No direct CO2 emissions during hydrogen production.
-
High-purity solid carbon that can be stored or used commercially.
-
Lower energy demand compared to electrolysis.
-
Compatibility with existing natural gas systems makes deployment easier.
However, the technology isn’t completely emissions-free. Upstream methane leaks—from extraction, processing, or transportation—can significantly increase greenhouse gas emissions. Methane has a global warming potential many times higher than CO2, so minimizing leaks is critical for keeping emissions low.
Thus, the process requires careful management of upstream methane leaks to ensure true low emissions. Also, methane supply chains must be monitored and controlled. Additionally, energy inputs must be optimized to maximize efficiency and minimize lifecycle CO2 emissions.
If successfully deployed, this technology could complement renewable-based hydrogen solutions and provide a scalable, industrial-ready pathway to cleaner hydrogen production. The Baytown demonstration plant will provide critical insights into operational efficiency, emissions management, and the commercial viability of methane pyrolysis.
Methane Pyrolysis vs. Other Hydrogen Methods
Energy Efficiency: Methane pyrolysis requires about 37.5 kJ of energy per mole of hydrogen, compared to 63.4 kJ for SMR and 285.8 kJ for water electrolysis. This shows methane pyrolysis is highly energy-efficient.
Lifecycle Emissions: Studies estimate methane pyrolysis produces 9–12 tons of CO2 equivalent per ton of hydrogen, depending on methane management and energy sources. SMR with carbon capture (CCS) has slightly higher emissions, while electrolysis emissions depend entirely on the electricity source. If powered by renewable electricity, electrolysis can achieve near-zero CO2 emissions, but grid electricity with fossil fuels increases emissions.
Full Lifecycle Benefits: Methane pyrolysis may also avoid some emissions linked to manufacturing and resource use for electrolyzers. Its efficiency and carbon byproduct make it a competitive low-carbon solution.
In summary, methane pyrolysis offers a balance between low emissions, energy efficiency, and economic feasibility. It competes well with SMR + CCS and is generally less energy-intensive than full electrolysis, though renewable-powered electrolysis has the lowest emissions if electricity is green.
BASF and Exxon’s Demonstration Plant to Validate Technology
BASF and ExxonMobil plan to build a demonstration plant at ExxonMobil’s Baytown Complex. This facility will produce up to 2,000 tons of low-carbon hydrogen and 6,000 tons of solid carbon annually. The project will validate the technology at scale and prepare it for commercial deployment.
This plant represents a key step toward making methane pyrolysis a practical solution for industrial hydrogen demand. By combining BASF’s chemical expertise with ExxonMobil’s experience in energy infrastructure, the companies aim to accelerate the global adoption of low-emission hydrogen.
A Strategic Leap for Clean Hydrogen Innovation
Moreover, ExxonMobil brings additional strengths to the partnership. The company owns the largest CO2 pipeline network in the U.S. and has extensive experience in fuels, chemicals, and low-carbon solutions. Combining this with BASF’s innovation in chemical processes makes the collaboration a powerful step forward for sustainable hydrogen production.
Overall, this partnership represents a major step in advancing low-emission hydrogen.
IEA predicts that low-emissions hydrogen production is set to grow significantly by 2030. Projects that are already operational or have reached final investment decisions (FID) are expected to produce 4.2 million tons per year (Mtpa) by 2030. It’s a fivefold increase compared with 2024.
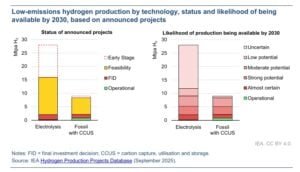
Although this is still below the ambitious targets set by governments and industry earlier in the decade, it would raise the share of low-emissions hydrogen from less than 1% today to around 4% of total hydrogen production by 2030.
This growth is similar to the rapid expansion seen in other clean energy technologies, such as solar PV. In addition, a new assessment of announced projects suggests that another 6 million tons of low-emissions hydrogen could become operational by 2030, provided effective policies are in place to support demand and secure offtake agreements.
As industrial hydrogen demand rises and decarbonization becomes urgent, methane pyrolysis is set to play a key role in the energy transition. By combining their expertise, BASF and ExxonMobil are positioning themselves at the forefront of low-emission hydrogen innovation.
The post BASF and ExxonMobil Team Up to Boost Low-Emission Hydrogen with Methane Pyrolysis appeared first on Carbon Credits.















