Green Chem., 2025, Advance Article
DOI: 10.1039/D4GC06348A, Paper
DOI: 10.1039/D4GC06348A, Paper
Yuka Abe, Tsuyoshi Yamada, Takuhei Yamamoto, Yukihiro Esaka, Takashi Ikawa, Hironao Sajiki
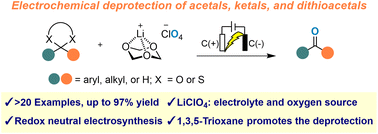
A novel electrochemical deprotection method for acetals, ketals and dithioacetals was developed, yielding carbonyl compounds. LiClO4 acts as both an electrolyte and oxygen source, while 1,3,5-trioxane enhances reaction efficiency as a Li activator.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
A novel electrochemical deprotection method for acetals, ketals and dithioacetals was developed, yielding carbonyl compounds. LiClO4 acts as both an electrolyte and oxygen source, while 1,3,5-trioxane enhances reaction efficiency as a Li activator.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry














