Green Chem., 2025, Advance Article
DOI: 10.1039/D5GC02358K, Communication
DOI: 10.1039/D5GC02358K, Communication
 Open Access
Open Access   This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
  This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.Momoko Ishii, Maria M. Paulsen, Remi A. Mellinghoff, Heather O. LeClerc, Ho Yin Tse, Hanno C. Erythropel, Julie B. Zimmerman, Paul T. Anastas, Darren S. Lee
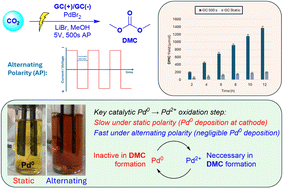
Alternating polarity enhanced redox-neutral electrochemical formation of dimethyl carbonate (DMC) from CO2 and methanol. Alternating the polarity was found to be necessary for efficient reoxidation of Pd0 → Pd2+ thus enabling higher yields of DMC.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
Alternating polarity enhanced redox-neutral electrochemical formation of dimethyl carbonate (DMC) from CO2 and methanol. Alternating the polarity was found to be necessary for efficient reoxidation of Pd0 → Pd2+ thus enabling higher yields of DMC.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry














